Expertise Therapeutic Areas
Oncology

Advancing Oncology Through Innovative Research
With 22 years of clinical trial experience and a passion for improving cancer treatments, Accelsiors is poised to accelerate breakthroughs in oncology research.
In recent years, there has been a shift from traditional organ-based studies focusing on cytotoxic chemotherapy to a more targeted approach. Biomarker-driven trials have emerged, utilizing molecularly targeted agents that interfere with crucial cell signaling mechanisms or immune responses to cancer in specific patient subsets. This paradigm shift emphasizes the significance of clinical trials in oncology.
Accelsiors recognizes and embraces the ongoing changes in scientific, methodological, practical, and patient-focused aspects of oncology trials.
We actively participate in the necessary transformation of these trials to stay at the forefront of advancements in the field. We understand that collaboration with key stakeholders is essential to establish a framework for next-generation clinical trials that balance operational efficiency, scientific impact, and value to patients.

Main regions are served by top professionals:

Accelsiors key facts in Oncology:
With cancer immunotherapy transforming outcomes, we help clients capitalize on cutting-edge approaches like combination therapies, neo-adjuvants, and biomarkers. Extensive Phase I-III experience in difficult therapeutic areas as rare diseases and proprietary methodology for trial acceleration, enable us to expedite oncology drugs development.
At Accelsiors, we believe collaborating across stakeholders can conquer cancer faster. By pursuing clinical partnerships in oncology, we can deliver life-changing treatments from bench to bedside.
Our Oncology Leadership Team has over 50 combined years of experience designing and operating cancer clinical trials from Phase I-III. They leverage deep therapeutic knowledge with practical operational expertise to drive trial success. Cross-functional collaboration with our wider Clinical Operations team, which has supported numerous global trials, ensures excellence in oncology protocols.
Oncology trials are supported by Accelsiors global team located in key locations worldwide, enabling knowledge sharing, local expertise at hand and rapid interventions.
Explore how our clinical trial expertise
can turn your vision into a reality.
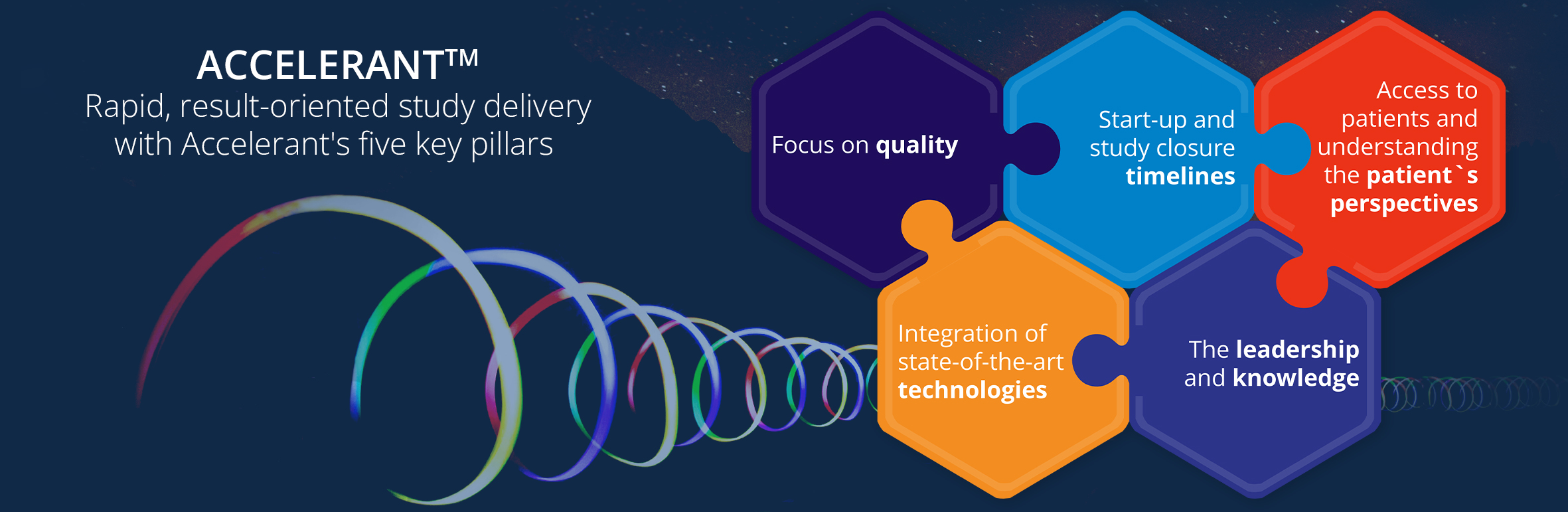
ACCELERANTTM
Let us support you
on your journey to
revolutionize cancer
therapy and improve
patient outcomes.
Our proprietary ACCELERANTTM methodology seamlessly integrates trial design, advanced data analytics, and risk forecasting to minimize obstacles. We identify process inefficiencies and implement optimized solutions, reducing trial timelines by an average of 30%.
Cutting-edge technology is crucial and we’ve developed and implement dedicated, proprietary modern solution WideScopeTM to expedite oncology trials. Robust SOPs and GCP-trained teams enable flexible management focused on accelerated approval timeframes.
We tap into academia through our Academic Research Organization, forging partnerships with leading cancer research institutions. By collaborating with top oncology investigators, we facilitate patient recruitment and operationalize cutting-edge clinical strategies.
At Accelsiors we believe clinical partnerships can conquer cancer faster. By collaborating across stakeholders, we can deliver innovative treatments from bench to bedside and transform outcomes for patients.
Our Oncology Experience

- Executed studies from phase I (first in human) to phase III (registration) trials
- Detailed database of highly qualified oncology sites worldwide
- Access to patients around the globe
- Significant experience in translational science in the field of oncology
- Depth of experience with population PK analysis in adult and pediatric population, including PK/PD modelling
- Vast experience in various oncology indications
- Experience in studies with intra-tumoral IMP administration
- Experience in pediatric oncology studies